Partnership Committee Meeting
Mon, 7/27/2020 - 9:00am - 12:03pm
Meeting Goals
- Review of HEAL preclinical pain portfolio.
- Critique realignment of HEAL therapy development program.
- Identify potential gaps or weaknesses in the realignment program.
- Recommend refinements to the re-alignment strategy to optimize successful project intake and program outcomes.
- Identify optimal metrics and benchmark targets for success at appropriate stages and for the overall program.
- Recommend optimal oversight approaches to evaluate, monitor, and criteria to progress or re-invest to ensure success of the program.
Agenda*
Presentations*
Introduction
A strategy to advance novel small molecules and biologics from preclinical to clinical development through a continuum of integrated projects through realignment of the HEAL Analgesics Development Program will be presented. The strategy brings together an interrelated set of programs that allow a more seamless transition through the drug development pipeline with a goal to deliver novel pain therapies to phase 2 clinical trials. The re-alignment will integrate some current drug development programs and leverage others and will provide broader access to NIH contracts to facilitate and accelerate the process of drug development. It will incorporate team-based science approaches and expert program oversight to monitor and evaluate progress and goal alignment in order to move promising projects forward through the pipeline. A goal is to shorten the time from discovery to human testing.
Highlights of the Re-aligned Analgesic Development Program
Program Oversight
An External Panel of Consultants will provide independent input and oversight on overall strategy and implementation of the program and projects. Late stage investigators will have access to domain expert consultants in areas such as medicinal chemistry, assay development, manufacture, and regulatory to help outline the development path towards clinical trials and identify appropriate go-no-go decision points. Expert consultants will work directly with investigators on selecting and testing models, developing and monitoring drug discovery efforts, candidate nomination, and program progress.
Early Stage Development Program
The Early Stage development program is designed to identify promising small molecule or biologic analgesics and corresponding assays and models ready for optimization and early clinical trials. Activities within this stage include confirmatory target validation studies, assay development and screening, rigorous efficacy studies including PK/PD/ADME, and end points and animal model development. Two components are proposed: 1) A multidisciplinary team-based approach in which each team would focus on a pain type, condition or promising molecular mechanism/target for development and engage in research to develop appropriate assays, starting point for therapeutic optimization, and a path forward. 2) Planning awards to encourage teambuilding and demonstrate the feasibility of high-risk targets including constructing preliminary data for targets, assays, and models and validate initial findings to build confidence to move into a Team project.
Late Stage Development Program
The Late Stage development program is designed to identify a clinical candidate and advance it to readiness for phase II clinical trials. The Late stage analgesic development program will provide researchers with resources that are not readily available to academic and small business organizations. It will encompass the process of therapeutics development including lead optimization to identify a development candidate, manufacture and IND-enabling toxicology studies, and Phase I clinical trials. The approach is to expand the capacity to provide resources to investigators with a promising validated asset. The vision for this stage is to de-risk therapeutic candidates to the point that they can be adopted by industry and advanced to help patients.
The Early and Late Stage Development Programs will leverage other NIH resources
Preclinical Screening Platform for Pain (PSPP): therapeutics developed through the early stage team-based programs and preclinical candidates developed during late stage programs can be tested through PSPP. Additionally, PSPP can provide access to large animal efficacy studies and independent verification of in vivo efficacy studies.
NCATS: Investigator access to NCATS intramural assay development programs to develop/test novel human cell-based assays and support late stage development and specific research contracts such as toxicology and manufacturing.
EPPIC Net: access to clinical expertise to investigators in the early and late stage programs to support targeted biomarkers and endpoint identification for analgesic development and clinical stage testing of specific targets and pain populations.
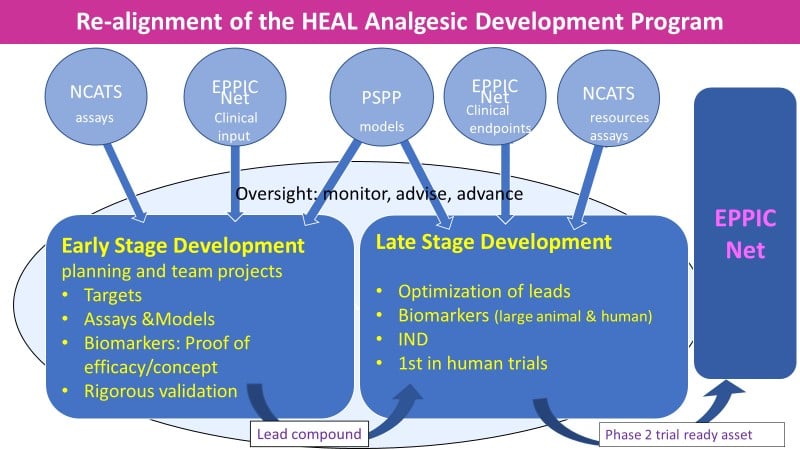
The graphic provides highlights of the Early and Late Stage Development Programs which integrate current HEAL programs on analgesic development (target validation RFA, optimization RFA, Biomarker Program) and other NIH programs (PSPP, NCATS intramural resources, EPPIC Net) that will be leveraged by the realigned Therapeutics Development Program to enhance the program.
* This document is in the process of being made compliant with Section 508 accessibility requirements.
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services
