Overview
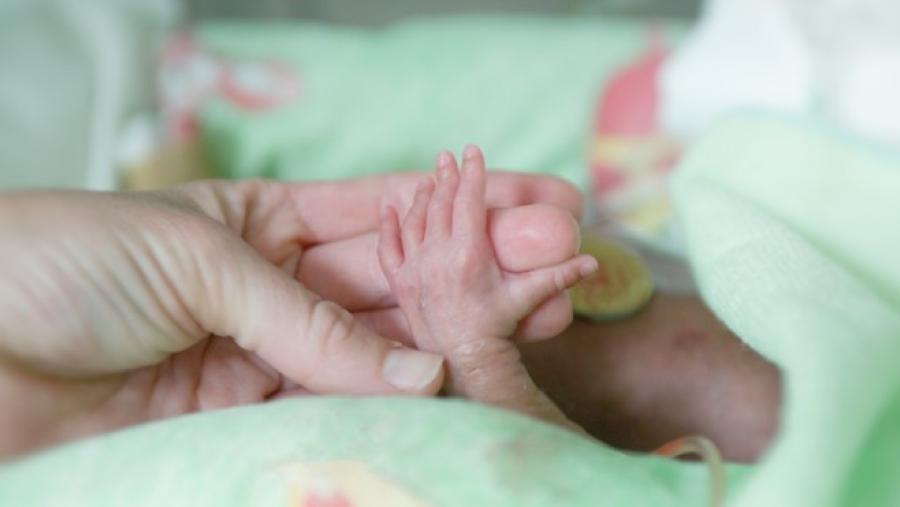
From 2004 to 2014, the incidence of neonatal abstinence syndrome and neonatal opioid withdrawal syndrome increased fivefold among infants covered by Medicaid in 46 states. Finding the best approaches to address the medical and social needs of these children is critical for the future health of the country.
Open Funding Opportunities
There are no Open Funding Opportunities at this time.Research Programs
The Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, expanded the Advancing Clinical Trials in Neonatal Opioid Withdrawal (ACT NOW) study. This research aims to inform clinical care for infants born with neonatal opioid withdrawal syndrome (NOWS). ACT NOW conducts clinical trials to determine best clinical practices for treating these infants with both drug-free treatment approaches and currently used medications.
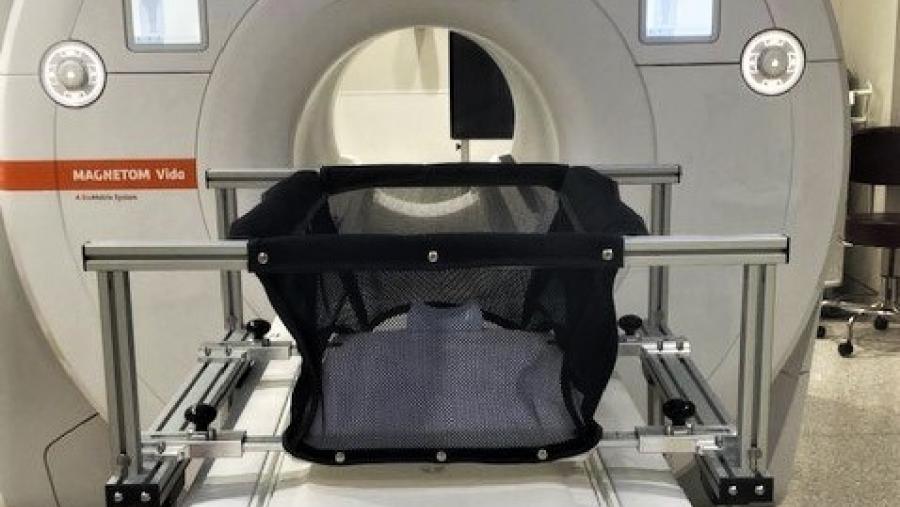
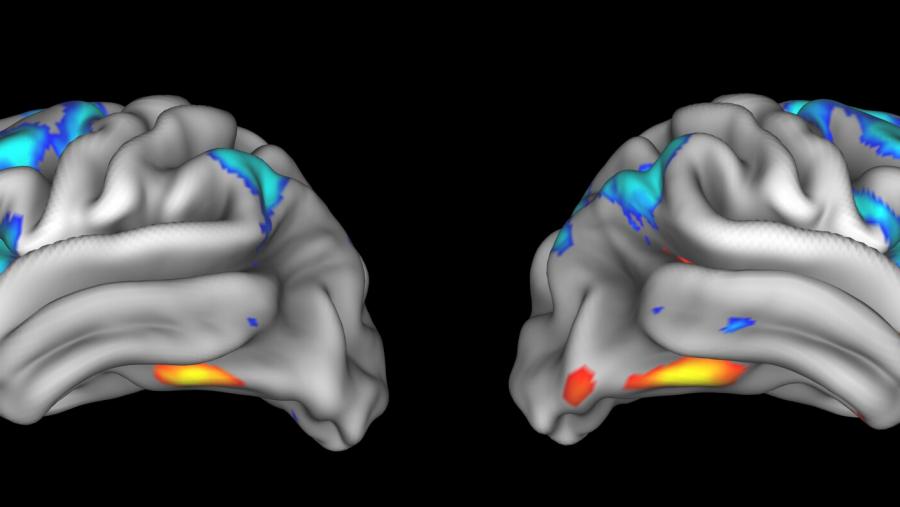
The Biology of Opioid Exposure During Pregnancy and Effects on Early Neuro-Behavioral Development program supports studies using human and animal models to examine how opioid exposure during pregnancy affects development of the placenta and fetal brain. The research will use cutting-edge technologies as well as longitudinal studies.
Understanding changes in brain and behavioral development resulting from early exposure to opioids will inform precision prevention for substance use disorders and mental illness. The HEALthy Brain and Child Development Study is building a large cohort of pregnant women from regions of the country significantly affected by the opioid crisis. The study will follow them and their children for at least 10 years.
Because infants grow and develop rapidly, their brains are susceptible to outside influences, including drug exposure. Such effects are difficult to measure in traditional clinical visits. The Virtual Assessments to Understand Developmental Trajectories of Substance Use Exposure program develops and validates novel technologies that allow monitoring of cognitive development during infancy and early childhood in naturalistic, family settings. This research will benefit infants and families from historically underserved communities, including those living in rural areas.

Addressing the impact of opioids on women and children
Women and children bear a substantial part of the burden of opioid overuse in the United States. Opioid use during pregnancy can lead to neonatal opioid withdrawal syndrome, and both the mothers and babies may be at higher risk of opioid use and its consequences later in the life course, setting up intergenerational cycles of opioid overuse.
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services