Back Pain Consortium Research Program
Overview
The Research Need
Chronic low back pain is one of the most common forms of chronic pain among adults worldwide; according to the Global Burden of Disease Study 2010, it ranked highest in terms of years lived with disability among hundreds of conditions. National Health Interview Survey data indicate that 20 percent of adults in the United States reported “frequent” back pain and 28 percent experienced low back pain that lasted one or more days during the previous three months. Current chronic low back pain treatment options are ineffective, which has led to an increased use of opioids.
About the Program
The Back Pain Consortium (BACPAC) Research Program is a translational, patient-centered effort to address the need for effective and personalized therapies for chronic low back pain (cLBP). It will examine biomedical mechanisms within a biopsychosocial context by using interdisciplinary methods and exploring innovative technologies.
The BACPAC Research Program has three main goals:
1. Develop a state-of-the-art model for cLBP.
Based on the current state of knowledge, this model will integrate biological, biomechanical, psychosocial, and other processes; test and re-test the implied relationships between those factors and cLBP; and adapt using data generated by or available to BACPAC.
2. Identify factors that are predictive of treatment effectiveness for well-defined patient subpopulations.
BACPAC will identify and test treatments targeting specific pathways implied by the theoretical model using data generated by BACPAC or existing datasets, conduct studies to elucidate interventional phenotypes, and test novel interventions and phenotyping approaches.
3. Conduct a large-scale collaborative clinical study (CCS) through which the consortium will develop an algorithm for multi-modal interventions for individuals with different cLBP phenotypes.
Incorporating research from BACPAC, HEAL, and other studies, the CCS will address novel questions regarding phenotypic factors influencing treatment effectiveness and define optimal treatment strategies for patients based on phenotypic characterization and intervention sequence or bundling.
A BACPAC Stakeholder Board composed of patients, providers, professional societies, payors, and industry, federal, and government-sponsored organizations provides ongoing feedback about the development and implementation of the CCS.
Open Funding Opportunities
There are no Open Funding Opportunities at this time.Contact
Current Trials
The Biomarkers for Evaluating Spine Treatments (BEST) clinical trial, part of the BACPAC Research program, aims to find links between a broad range of personal traits, known as biomarkers, and the effectiveness of four existing, proven treatments for chronic lower back pain – which can help doctors make better, more personalized treatment recommendations in the future. Participants receive one to two of these treatments, which include: acceptance and commitment therapy; duloxetine; enhanced self-care; and evidence-based exercise and manual therapy.
The BEST study is taking place in clinical sites across the United States and is using a precision medicine approach to help researchers learn the best treatment for patients with particular biomarkers. Precision medicine aims to understand how a person’s genetics, environment, and lifestyle can help determine the best approach to prevent or treat disease. To learn more about the study, please visit the BEST webpage.
Upcoming Events
Please check back for more upcoming events.
Back Pain Consortium (BACPAC) Research Sites
To date, through the Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, NIH has funded 13 awards for this program, totaling $130.8 million.
View Other Research Programs in This Focus Area
- Discovery and Validation of Biomarkers, Endpoints, and Signatures for Pain Conditions
- Early Phase Pain Investigation Clinical Network (EPPIC Net)
- Integrated Approach to Pain and Opioid Use in Hemodialysis Patients (HOPE)
- Integrative Management of Chronic Pain and OUD for Whole Recovery (IMPOWR)
- Pain Management Effectiveness Research Network (ERN)
- PRagmatic and Implementation Studies for the Management of Pain to Reduce Opioid Prescribing (PRISM)
Mechanistic Research Centers (MRCs) collect data on patients through clinical and other research efforts in an effort to identify specific traits unique to chronic low back pain patients. The goal of this research is to help explain how these individual characteristics affect chronic low back pain and to identify new treatments.
University of California San Francisco
Jeffrey Lotz, PhD
The Core Center for Patient-centric, Mechanistic Phenotyping in Chronic Low Back Pain (REACH) is a BACPAC Mechanistic Research Center that conducts translational and clinical research to clarify biopsychosocial mechanisms of chronic low back pain – the interconnection between biology, biomechanics, psychology, and socio-environmental factors – which will be foundational for new diagnostic and therapeutic strategies. For more information, visit https://www.bacpac-reach.org/
University of Michigan Ann Arbor
Daniel Clauw, MD, Afton Hassett, PsyD
As part of the BACPAC initiative, we will enroll patients with chronic low back pain in a clinical trial and follow them longitudinally as they try different evidence-based therapies as they might in routine clinical care. The goal of our study is to link patient characteristics (i.e., pain mechanisms, biomarkers) to treatment responses – the holy grail of personalized medicine.
University of Pittsburgh
Gwendolyn Sowa, MD, Nam Vo, PhD
The LB3P Mechanistic Research Center--led by co-Principal Investigators, Drs. Gwen Sowa and Nam Vo--is an innovative, multidisciplinary collaboration of investigators at the University of Pittsburgh and University of Pittsburgh Medical Center. The Center will collect data from three key contributors of chronic low back pain: biological, biomechanical, and behavioral markers. The data collected will be analyzed to perform in-depth phenotyping of patients with chronic low back pain with the goal of characterizing patients and directing targeted, improved treatments.
Tech Sites create, test, and distribute technology and methods to test, quantify, and relieve chronic lower back pain.
Brigham Young University
Anton Bowden, PhD, PE
At its fundamental core, the spine was built to move, and the way it moves is a direct window into spinal health. Our research team is dedicated to improving chronic lower back pain (cLBP) outcomes through the use of unique, inexpensive, screen-printable, elastomer-based nano-composite piezoresponsive sensors that we invented, and which we are integrating into a SPInal Nanosensor Environment (SPINE Sense System). By identifying and characterizing the mechanical phenotypes of dysfunctional spinal motion, our technology will provide an objective, quantitative platform for diagnosis, monitoring, and follow-up assessment of cLBP which can be implemented both in the clinic, as well as during activities of daily living.
Harvard University
Conor Walsh, PhD
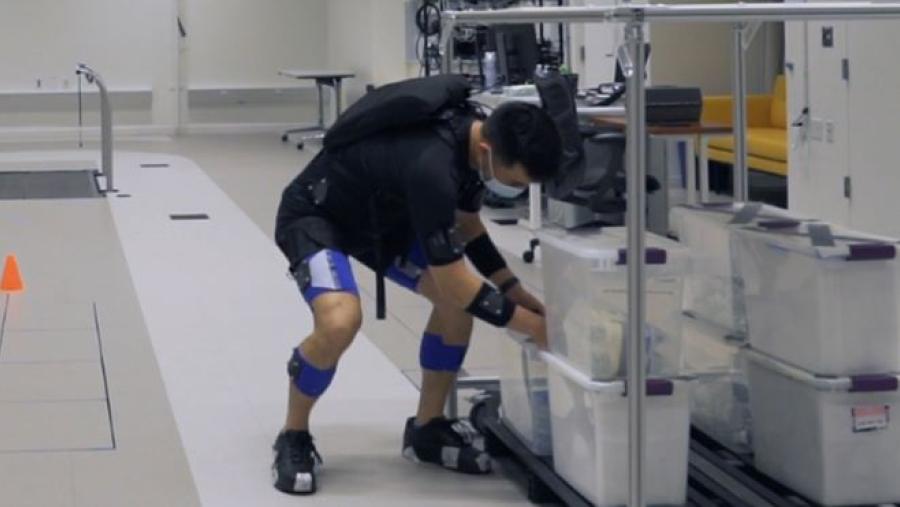
Harvard University and Boston University are developing robotic apparel to reduce the risk of back injury in occupational settings where workers perform physically strenuous tasks as well as aid in recovery for individuals with lower back pain (LBP) undergoing rehabilitation. The technology integrates motion sensors, electromechanical actuation components, and adaptive control algorithms into lightweight and comfortable functional apparel that aids the movement of the wearer. Laboratory-based biomechanics experiments with healthy subjects will evaluate the ability of the device to reduce muscular exertion and fatigue during lifting tasks and studies with individuals with LBP will assess the technology’s ability to integrate and assist with occupational therapy procedures.
Massachusetts General Hospital
Hsiao-Ying Monica Wey, PhD
Histone deacetylase (HDAC) enzymes are key components regulating gene expression in response to life experience and have recently been associated with the development and maintenance of pain. Using neuroimaging techniques, we will evaluate HDAC density in the brain of lower back pain patients to determine if HDAC is a biomarker capable of differentiating subtypes of low back pain and monitoring progression of pain.
Ohio State University
William Marras, PhD, CPE
The Ohio State University Spine Research Institute’s effort aims to develop and validate a digital health platform that phenotypes patients based on quantitative evaluations of spine motion, patient reported outcomes, Electronic Medical Records, and other available meta data. Collectively, the ability of this platform to phenotype patients will enable clinical decision-making on personalized treatments to improve low back pain patient outcomes.
University of California San Francisco
Aaron Fields, PhD, Roland Krug, PhD
Endplate pathology commonly associates with chronic low back pain (cLBP), but methods for identifying cLBP patients in whom endplate pathology is a contributing factor and for selecting patients for non-addictive and minimally invasive treatments remain qualitative, subjective, and underdeveloped. The overarching goal of this project is to further develop and validate novel, quantitative MRI measures of endplate pathologies. Accomplishing this goal will improve diagnosis of endplate pathologies in cLBP patients and provide tools that would be broadly applicable for identifying phenotypes, discovering pain mechanisms, uncovering treatment targets, and selecting patients for treatment.
University of California San Francisco
Sharmila Majumdar, PhD
While the roles of disc composition, vertebral endplate integrity, bone remodeling at the facet joints, nerve inflammation, muscle composition, area and spinal stability in chronic lower back pain (cLBP), have been extensively studied, more often in isolation, etiology of cLBP often involves multiple sources, requiring assessment of metrics from multi-system and multi-modal medical imaging data. Long imaging times (especially with back pain) for quantitative imaging and non-standardized image interpretation are long-standing challenges in the field today, limiting the clinical translational utility of medical imaging. The current variations in derived metrics would be significantly impacted by automated decision support tools. This Technology Site at UCSF will undertake technical development of an integrated platform to provide accelerated quantitative imaging, standardized quantitation of degenerative characteristics, spinal deformities, musculoskeletal models, and the ability to enhance their relationships with Patient Reported Outcome Measures (PROMs), with functional brain metrics.
University of Utah
Viola Rieke, PhD, Lubdha Shah, MD
The Focused Ultrasound Group as part of the Utah Center of Advanced Imaging and Research aims to improve patient care by exploring, developing, and translating new, less invasive treatment options using focused ultrasound (FUS) technology. The goal of our project is to develop a completely non-invasive, precise and durable treatment option for low back pain with FUS neuromodulation of the dorsal root ganglion. The lower risk associated with FUS, compared to more invasive procedures, will result in fast translation to humans and has the potential to replace current invasive or systemically detrimental treatment modalities.
Phase II Studies test the safety and value of alternative medicine, non-addictive drugs, and devices that relieve chronic lower back pain and help patients carry out activities of daily living. The new therapies tested in these trials will use biology, psychology, and information on social factors connected to chronic lower back pain.
Cedars-Sinai
Brennan Spiegel, MD, MPH
CS-CORE is a full-service health services research (HSR) laboratory. Lab members have published extensively in the fields of healthcare quality and safety, patient-reported outcomes (PROs), medical decision-making, health economics, and medical informatics. The multidisciplinary team includes clinicians, social scientists, epidemiologists, health economists, psychometricians, biostatisticians, and computer scientists working across medical, surgical, and behavioral specialties. In addition to its focus on traditional HSR methods, CS-CORE has a robust program in digital health science research, including federally funded programs using clinical decision support (CDS), mobile health (mHealth), wearable biosensors, smartphone applications, social media analytics, and electronic health record patient portals. The lab launched a major program in medical virtual reality (VR) in 2015 that has gained international attention and media coverage after treating over 3,000 patients with therapeutic VR. Our BACPAC study, Randomized-controlled trial of virtual reality for chronic low back pain to improve patient-reported outcomes and physical activity, builds on this experience. This study will test the effectiveness of an evidence-based VR therapy program as a non-pharmacological supplement to management of chronic lower back pain (cLBP). Study participants will be randomized to receive one of three VR programs: skills-based VR, distraction VR, or sham VR. In addition to a VR headset, all participants will receive a Fitbit Charge 3 watch. They will be followed for 90 days and complete PRO questionnaires to assess functional status, pain levels, and use of pain medications (including opioids). The primary outcome will be measured using the 8-item PROMIS Pain Interference questionnaire at 4 weeks.
University of Pittsburgh
Ajay Wasan, MD, MSc
The University of Pittsburgh is conducting a randomized trial to test whether the combination of anti-depressants and an enhanced fear avoidance rehabilitation protocol is more effective than each treatment alone at relieving pain, improving function, combating depression, and preventing opioid misuse in chronic lower back pain (cLBP) patients with high negative affect (comorbid depression or anxiety disorders). This multimodal combination approach of pharmacotherapy and behavioral therapy is novel to the field and has the potential to shift current treatment paradigms.
The Data Integration, Algorithm Development and Operations Management Center (DAC) coordinates the BACPAC Research Program's activities, ensures communication and accountability, and manages a registry of patient-reported outcomes. The DAC will also provide support for the design, development, implementation, monitoring, and analysis of clinical research that the program conducts. Using data from the BACPAC Research Program’s clinical studies, the DAC will develop patient-centered algorithms to predict optimized therapeutic interventions and lead to an integrated model of chronic lower back pain.
University of North Carolina at Chapel Hill
Lisa LaVange, PhD, Anastasia Ivanova, PhD
The DAC is located at the Collaborative Studies Coordinating Center (CSCC) at the University of North Carolina at Chapel Hill. The DAC guides and coordinates activities and communication among the other 12 BACPAC study sites and is responsible for developing and hosting a secure, cloud-based, scalable data management and analysis platform to facilitate data sharing and collaborative analysis of data collected by the Consortium. The DAC will collaborate with Consortium members to conduct system level analysis for BACPAC generated multidimensional datasets to refine the Consortium’s model of lower back pain (LBP) to develop patient-centered algorithms for prediction of optimized therapeutic interventions. In collaboration with the BACPAC Clinical Management Committee, the DAC Adaptive Design Expert Group will design one or more collaborative studies, to be conducted by the Consortium, for which the DAC will serve as the central data coordinating and analysis center.
University of Utah
Julie M. Fritz and Daniel Rhon
Most patients considering spine surgery have experienced many years of back pain and rates of pre-surgical opioid use are high. Pre-surgical opioid use is predictive of worse functional outcomes, higher complication rates and greater costs after surgery. The idea behind this project is that surgical outcomes can be improved, and risk for post-surgery opioid use reduced through comprehensive, enriched pain management designed to enhance patients’ ability and confidence in self-managing pain. This project includes a one-year planning phase and a two-year clinical trial, and expands the scope of an ongoing clinical trial in the Military Health System, investigating similar interventions for patients pursuing non-surgical management for chronic back pain.
Dataset Requirements and Recommendations
The Back Pain Consortium (BACPAC) Minimum Dataset defines a collection of core data elements to be collected in all longitudinal BACPAC studies involving chronic low back pain patients. Longitudinal assessments are performed at both baseline and 3-month follow-up visits (3-month +/- 2 weeks).
BACPAC Definition of cLBP (BACPAC/2014 Task Force Crosswalk)
Treatment Categories Questionnaire
BACPAC Minimum Dataset: Required Baseline Demographic and Outcomes Measures
This document contains domains and instruments for the longitudinal assessments in the BACPAC Minimum Dataset.
Where applicable, core data elements of the BACPAC Minimum Dataset are taken from previously validated instruments (e.g., PROMIS measures). The BACPAC Minimum Dataset is an expanded version of the HEAL Initiative Core Data Elements. In addition to the longitudinal assessments, the BACPAC Minimum Dataset includes a collection of demographic and baseline characteristic core data elements, which are administered to study participants at baseline only.
BACPAC Biobehavioral Research Psychosocial Questionnaires and QST Recommendations
This document includes recommendations for the following domains:
- Psychosocial Recommendations
- Social Adversity and Support
- Other Optional Domains
- Quantitative Sensory Testing Recommendations
BACPAC Comorbidity Assessment Guidelines
This document includes recommendations for how comorbidities should be assessed in the clinic and via electronic health records. The document recommends ICD-10 codes to be used with the Charlson Comorbidity Index and adds a question regarding COVID diagnosis.
Sharing Common COVID-19-related Challenges and Solutions
During the BACPAC Spring 2021 Bi-Annual Meeting (March 1-2, 2021) members of the consortium joined together virtually for collaboration on many topics, including COVID-19-related challenges and solutions. Principal Investigators (PIs) from the Mechanistic Research Centers (MRCs), Phase 2 Trials, and select Technology Sites were asked to give a brief presentation on the challenges their sites have faced during the COVID-19 pandemic and share solutions that they have found. The presenters on this topic are sharing these solutions in these presentation decks with the goal of helping others who are facing similar challenges. View each presentation deck below.
- COVID-Related Challenges and Solutions: UM MRC
- Nonpharmacologic Pain Management for Lumbar Surgery
- COVID-Related Challenges and Solutions: Experiences from Cedars-Sinai Conducting a Remote Clinical Phase II Trial
- COVID-related challenges: Tech site experiences
- University of Pittsburgh’s MRC LB3P Update on COVID-related Barriers
- Brigham Young University – Utah
- Cedars-Sinai Medical Center – California
- Harvard University – Massachusetts
- Massachusetts General Hospital – Massachusetts
- Ohio State University – Ohio
- University Of California, San Francisco – California
- University Of Michigan – Michigan
- University Of North Carolina, Chapel Hill – North Carolina
- University Of Pittsburgh – Pennsylvania
- University Of Utah – Utah
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services