Overview
Expanded treatment options for opioid use disorder are needed to promote long-term recovery. The Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, is accelerating the development of treatments for all aspects of the opioid addiction cycle, including progression to chronic use, withdrawal symptoms, craving, relapse, and overdose.
Open Funding Opportunities
There are no Open Funding Opportunities at this time.Research Programs
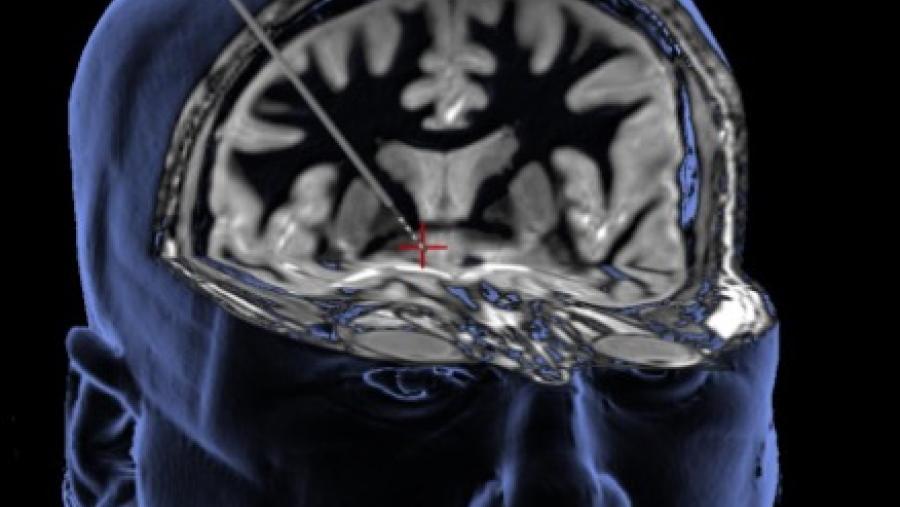
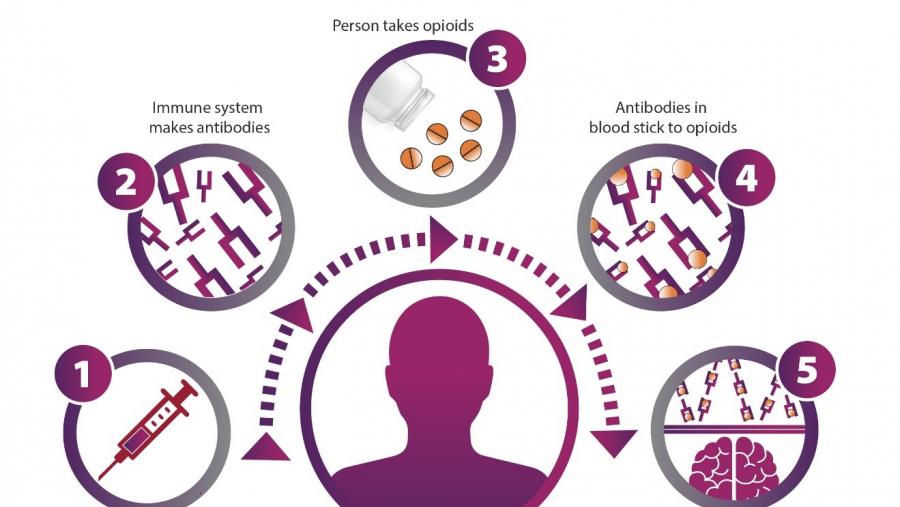
The Development of Novel Immunotherapeutics for Opioid Addiction program is developing a coordinated, multidisciplinary consortium to develop anti-opioid vaccines and monoclonal antibodies and test them in clinical trials. These immunotherapies bind specifically to opioid targets, reducing their effects and offering protection from overdose.
The Focusing Medication Development to Prevent and Treat Opioid Use Disorder and Overdose program supports the development of new targets and formulations of existing medications to treat opioid use disorder and stimulant use disorders. This research also supports development of longer-duration formulations to counteract opioid overdose, interventions for respiratory depression, and novel medications to treat progression to chronic use, withdrawal, craving, and relapse.
Long-term use of oral formulations of buprenorphine has been reported to cause oral problems ranging from dental caries to tooth loss. The Oral Complications Arising From Pharmacotherapies to Treat Opioid Use Disorder program supports basic research to understand how these problems arise. Clinical studies will also test how often and how quickly buprenorphine causes or worsens oral problems, as well as determine which other factors affect oral health in people taking buprenorphine.
The opioid crisis is constantly evolving: New and increasingly dangerous drugs are entering the supply. The Rapidly Assessing the Public Health Impact of Emerging Opioid Threats program supports research to develop and distribute validated analytical methods to detect emerging drug threats in clinical and forensic settings. These tools can be used to isolate drug use patterns so first responders can offer help.
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services