Overview
The Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, supports a wide range of programs to develop new or improved prevention and treatment strategies for opioid addiction and co-occurring conditions such as mental illness and polysubstance use. Programs are developing and implementing prevention and treatment strategies for a range of at-risk populations and in various settings. This research is also developing strategies to understand and monitor biological and social factors that drive disease progression, as well as defining approaches to improve treatment retention.
Open Funding Opportunities
Research Programs
This program will design and test a primary care setting-based, multidisciplinary treatment model to serve patients with unhealthy use of opioids and alcohol, stimulants, and/or sedatives. Researchers will first plan, develop, and define a treatment model and pilot test its feasibility and acceptability.
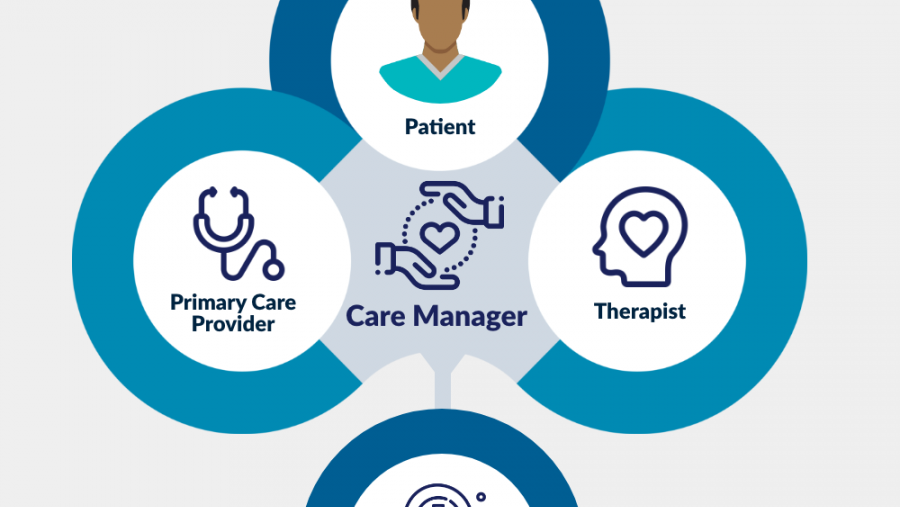
Research shows that collaborative care is effective for treating mood and anxiety disorders, but more research is needed to demonstrate its usefulness for treating opioid use disorder. The Optimizing Care for People with Opioid Use Disorder and Mental Health Conditions program tests the adaptation, effectiveness, adoption, scalability, and sustainability of collaborative care for individuals with opioid use disorder and co-occurring mental health conditions using integrated treatment models in primary care settings.
The Optimizing the Duration, Retention, and Discontinuation of Medication Treatment for Opioid Use Disorder program supports research to define the optimal length of medication treatments approved by the U.S. Food and Drug Administration for opioid use disorder (methadone and buprenorphine), taking into account various patient populations and treatment settings.
The Preventing Opioid Use Disorder program supports prevention research focused on underserved populations that experience health disparities and centered around four strategic areas: identifying risk, studying the role of social determinants and policy, developing effective interventions, and disseminating and implementing sustainable, scalable prevention services for populations with risk for opioid misuse.
The Subthreshold Opioid Use Disorder Prevention (STOP) Study aims to help researchers better understand how to define, identify, and intervene in the management of opioid misuse in primary care settings among individuals with low-severity opioid use disorder.
The Sleep Dysfunction as a Core Feature of Opioid Use Disorder and Recovery program supports research using genomic, molecular, pharmacological, and clinical approaches to better understand sleep and circadian factors relevant to addiction and how these factors influence one another. This research could open new avenues for prevention and treatment of opioid use disorder.
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services