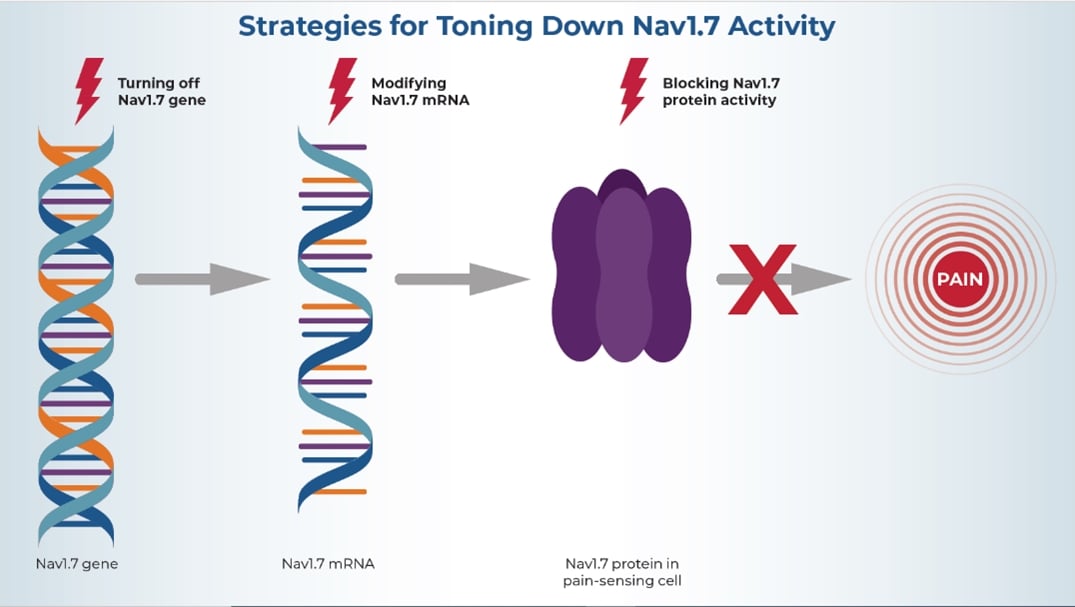
HEAL-funded research is seeking to disrupt the production and action of Nav1.7 at various stages to stop it from helping to transmit pain signals.
Every year, health care providers prescribe millions of doses of pain medications. Yet these medications don’t work for everyone; may not fully control the individual’s pain; and come with their own health risks, such as addiction. New safe, effective, and non-addictive pain medications are urgently needed for the 50 million Americans living with chronic pain, half of whom have severe pain that significantly impacts their daily living. Over the past 5 years, no innovative pain medications with new targets have been approved other than for migraine, but researchers are hopeful this will change.
Scientists funded by the NIH Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, are on the quest for new, non-addictive pain medications. One target that they have evaluated in recent years is a protein called Nav1.7, which is thought to be crucial for sending pain signals from pain-sensing cells to the spinal cord and brain. Initial validation of Nav1.7 as a target to treat pain came from genetic studies showed that some people with congenital chronic pain have an overactive Nav1.7 protein, whereas individuals who were born unable to feel pain have defective Nav1.7 proteins.
Researchers hope that by turning off or toning down Nav1.7 activity, they can silence pain-sensing cells and thus relieve pain. Various teams are pursuing different approaches to achieve that goal. Some of these approaches seek to block the activity of the Nav1.7 protein, others target the Nav1.7 gene or molecules called messenger RNA (mRNA) that the cell needs to produce Nav1.7 protein. Many small businesses supported by the NIH HEAL Initiative’s Small Business Programs are leading the way.
Blocking Nav1.7 Activity
Several research teams aim to turn down Nav1.7 activity—either with small molecules that can easily enter cells and block (or inhibit) Nav1.7 function, or with monoclonal antibodies (lab-made immune system proteins) that can specifically target and attach to Nav 1.7 protein and stop it from working. This can be challenging, especially when using small-molecule inhibitors, because there are different types of Nav proteins with similar structures. As a result, some molecules that act on Nav1.7 may also block other Nav proteins that help control critical body functions like breathing and heart rhythm. This may lead to serious safety risks.
John Mulcahy, Ph.D., and his team at SiteOne Therapeutics, Inc., have discovered a series of compounds that target only Nav1.7. And, unlike other inhibitors, their compounds block Nav1.7 whether it’s currently active or not. The researchers hope these compounds can mirror the lack of pain sensation that’s seen in people with a defective Nav1.7 gene. Initial studies in animals have yielded promising results. To advance their research, SiteOne Therapeutics is collaborating with the pharmaceutical company Vertex to evaluate Nav1.7 blockers and, if the studies are successful, bring them to the market. Additionally, Mulcahy’s team is exploring the effectiveness of molecules targeting a related protein, Nav1.8, in the treatment of acute and chronic pain.
Modifying Nav1.7 mRNA
When a cell needs to produce a protein like Nav1.7, it first produces mRNA molecules that carry the building instructions stored in the DNA to the cell’s “protein assembly line.” Thus, if the building instructions of the mRNA are changed, the resulting protein will also be altered.
The goal of Dr. Joshua Rosenthal and his colleagues at the Marine Biology Laboratory at Woods Hole is to modify, or edit, Nav1.7 mRNA so that it creates less active Nav1.7 protein. They are developing chemical compounds that will change the mRNA directly in the cells that naturally produce Nav1.7. As a result, these cells produce less active Nav1.7, which the researchers hope will reduce pain signals. “Using RNA editing as a treatment for pain is entirely novel,” says Rosenthal. “For pain management and treatment, we want to achieve long-lasting effects but without permanently changing the system. RNA editing allows for this.”
The team is currently testing their first set of mRNA-editing reagents in human and mouse nerve cells grown in the laboratory. They have also created genetically modified mice that carry more human-like Nav1.7 channels for the next stage of testing their reagents. If their approach can effectively modify signal transmission in cultured nerve cells and reduce pain perception in the modified mice, further studies in humans could follow.
Turning Off the Nav1.7 Gene
Ana Moreno, Ph.D., and her team at Navega Therapeutics, Inc., are pursuing the third avenue of trying to reduce Nav1.7 activity—they want to lower the activity of the Nav1.7 gene. Moreno explains, “By targeting Nav1.7 at the DNA level, we can achieve better pharmacokinetic prospects than RNA-targeting strategies and better specificity than protein-targeting approaches.” However, the team doesn’t want to mutate the DNA sequence, and thus Nav1.7 production, permanently, because that may have unwanted effects. Instead, they are using so-called epigenetic modulation: Cells can temporarily modify the DNA accessibility to turn a gene “on” or “off,” depending on the needs of the cell or organism. The researchers are trying to mimic that process to downregulate Nav1.7. That way, when circumstances change, the modifications can be reversed to adapt gene activity.
Moreno began developing the strategy, which uses a gene therapy compound that modulates Nav1.7 gene activity, as part of her Ph.D. dissertation. After getting promising results in animal models of various types of chronic pain, she and her colleagues founded Navega Therapeutics to continue development of the gene therapy. One challenge they face is that they must tailor the compound for each type of organism, but they have now identified a candidate drug that can target the Nav1.7 gene in human cells. The next step, which they are hoping to initiate soon, is to test this compound in people with chronic pain.
Which of these approaches will be successful? It’s too soon to tell, as most of these projects are still in early stages and they have to overcome side effects related to Nav 1.7 blockages and the autonomic nervous system. However, by supporting these and other projects that all focus on Nav1.7 but use very different strategies, the NIH HEAL Initiative is increasing the odds that this molecule may become a valuable new target for helping people with different chronic pain conditions.
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services
