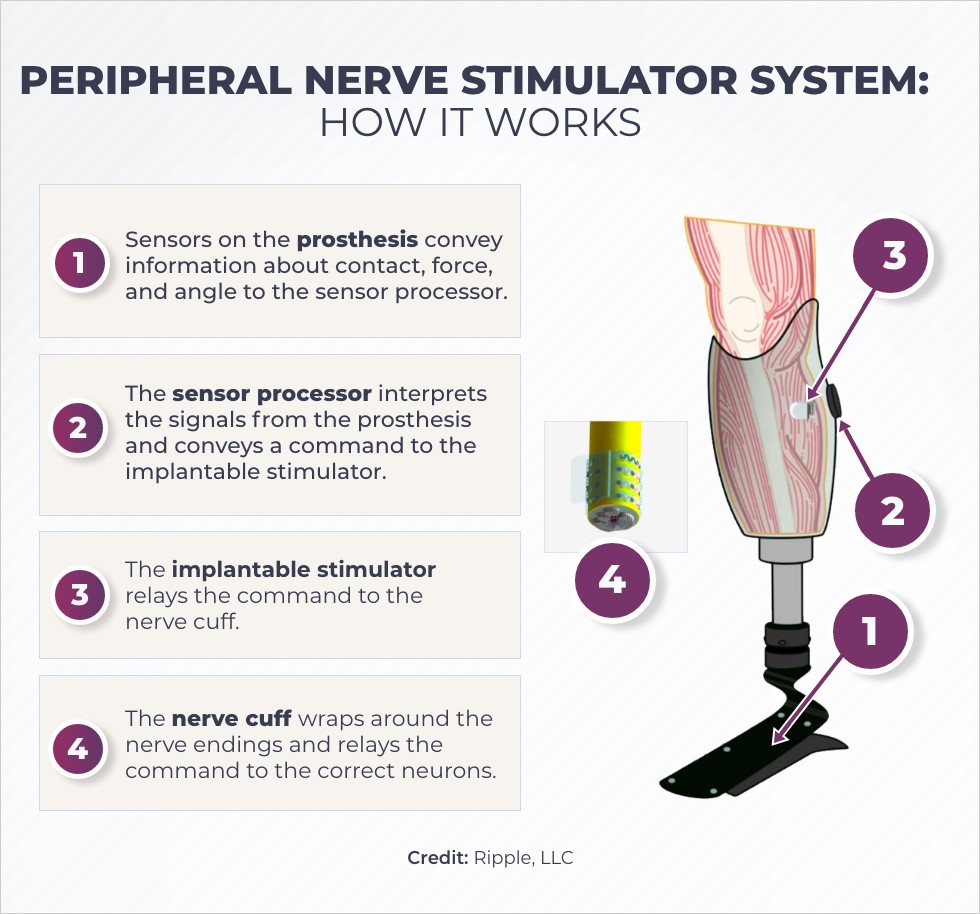
Illustration of how the peripheral nerve stimulator system works. Credit: Ripple, LLC.
Using controlled electric signals, a novel device restores sensation after the loss of a limb and could reduce phantom limb pain, a condition that afflicts up to 80% of people with amputations. It also might improve the stability of limb prostheses and lessen the need for opioid medications.
Ripple LLC, a Utah-based company, is developing the device, a neural stimulation system, with funding from the NIH Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, under its program to translate discoveries into effective devices to treat pain.
“Many people report that phantom limb pain is more debilitating than the loss of the limb itself,” said Daniel McDonnall, Ph.D., the chief scientific officer of Ripple and president of a related company, Sync Bionics. The company’s device is designed to electrically stimulate the nerves that remain in the residual limb to decrease phantom limb pain for people who have had an amputation. When paired with a prosthesis, or artificial limb, Ripple’s sophisticated device will enable users to sense the prosthesis as though it is a part of their body.
The neural stimulation system is currently undergoing testing in preparation for a clinical trial that will implant the device in people with below-the-knee amputations.
The NIH HEAL Initiative is building on recent scientific insights into the human nervous system and pain pathways with its support of research aimed at developing effective new ways to treat pain without opioids.
One group with a great need for new pain treatment options is the 1.6 million people in the United States who are living with the loss of a limb — all or a portion of an arm or leg — because of a medical condition or injury. Due to the rising number of people who have diabetes, a major cause of amputations, researchers estimate that the number of amputations could grow to as many as 3.6 million by 2050.
More than 7 in 10 people who have had an arm or leg amputated have pain in the lost limb, which they perceive as still present. That’s called phantom limb pain. Nearly 4 in 10 have severe pain, making it hard to sleep, work, and take part in family and social activities. Many amputees also report depression and anxiety linked with phantom limb pain.
Those who have phantom limb pain are often prescribed medications, including opioids, but opioid medications are effective in only about half of these patients. Opioids can also have serious side effects, including the risk of addiction, that make coping with the lost limb and the pain even more difficult.
Restoring the nervous system’s communication
Peripheral nerves lie outside the brain and spinal cord and transmit messages between the brain and the arms and legs. When a limb is amputated, the peripheral nerves are cut, but the residual portions of the nerves in or at the stump of the amputated limb remain.
After an amputation, nerve cell (or neuron) connections are "rewired" and continue to exchange signals with the brain, where connections are reorganized. Scientists believe this process causes phantom pain. Research has shown that controlled peripheral nerve stimulation can manage phantom limb pain by restoring subtle sensations that the brain perceives as normal input coming from the missing limb.
“We want to restore the natural kind of signals, something that the central nervous system is used to receiving from the leg,” McDonnall said. “We are using much smaller signals to avoid overwhelming the nerve, and we’re trying to selectively stimulate only a few neurons at a time. For example, there is a particular neuron that tells you when your big toe is tapping in a particular area and in a particular way. That signal travels up your leg to the central nervous system. We’re trying to target those few neurons that send this sensation but not turn on all of the rest of them at the same time.”
The peripheral nerve stimulation system
The project includes development of an innovative technology called a nerve cuff, made from a flexible material that conducts electricity. Ripple will produce the nerve cuff using a 3-D printer. The nerve cuff electrodes form a dome over the residual part of the nerves. This dome softly dimples into the nerves, so that it touches them without causing damage.
The nerve cuff is just one part of the peripheral nerve stimulation system. The communication begins with sensors on the prosthesis which send information about its movement, such as force and angle, to the sensor processor placed on the leg. The sensor processor interprets this information and sends signals to the nerve stimulator that is implanted in the leg. The stimulator conveys the message to the nerve cuff, which stimulates the correct nerves in the leg to mimic normal sensation.
Together, the sensor processor, the stimulator, and the nerve cuff act as a sensory feedback system to provide consistent and controlled electrical stimulation based on the actual movements of the prosthesis.
Based on prior research, including from the Defense Advanced Research Projects Agency Hand Proprioception and Touch Interfaces (DARPA HAPTIX) program, this feedback loop is expected to reduce pain and make the prosthesis feel more like the lost part of the body. Using this integrated system, a person with a lower-limb amputation could, for example, sense their prosthetic heel and foot contact the ground when walking. The system is expected to provide better control of the prosthetic, reducing the risk of falls. This, in turn, produces important psychological benefits for the user.
Ripple will conduct the electrical, biochemical, and mechanical tests needed to determine whether the device is safe and effective, and that the system can recognize accurately and communicate sensations between the prosthesis and the residual nerves.
Based on these data, Ripple plans to submit an investigational device exemption (IDE) application to the Food and Drug Administration (FDA) to start human tests. Pending FDA approval, McDonnall hopes that the first-in-human testing of the implantable system will begin in early 2022.
For the in-human testing phase, the company will work with Giancarlo Barolat, M.D., of Presbyterian St. Luke’s Medical Center in Denver, to implant the peripheral nerve stimulator into five people who have undergone below-the-knee amputations. The devices will be tested for 1 year to evaluate safety and effectiveness.
Public-Private Partnerships to Benefit Patients
It can be a big leap, particularly for a small company like Ripple, to develop this new technology to produce new devices and get them to the patients who need them, McDonnall said.
A big challenge is that the market demand for the peripheral nerve stimulator is relatively small, making it difficult to attract private capital investment. Ripple has been able to leverage grants from NIH, DARPA, and others to build the core technologies and assemble a team to bring the device to the clinic.
“Without the support of NIH and programs like the NIH HEAL Initiative, it would be very difficult to develop the advanced technology that we need to build therapies for these patients,” McDonnall said.
Currently, there is no effective treatment for chronic phantom limb pain, despite its devastating effects on the quality of life of most people following an amputation. Although this patient population is small, for HEAL researchers and providers, a novel and non-opioid treatment can't come fast enough.
Stay Connected
Stay up to date on the latest HEAL Initiative research advances by subscribing to receive HEAL content directly to your inbox.

Find More Projects in This Research Focus Area
Explore programs and funded projects within the Preclinical and Translational Research in Pain Management research focus area.

 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services
