Native Collective Research Effort to Enhance Wellness (N CREW) Program - Questions and Answers (Q&A)
N CREW Program Background, Goals, and Structure
1. What are the goals of N CREW?
The N CREW Program focuses on three main goals that reflect priorities for addressing the opioid/drug overdose crisis identified in Tribal Consultations (in 2018 and 2022) and through community input:
- Support Tribes and Native American Serving Organizations (T/NASOs) to lead community prioritized research projects, including research elevating and integrating Indigenous Knowledge and culture.
- Enhance capacity within T/NASOs to conduct locally prioritized research by developing and providing novel, accessible, culturally grounded technical assistance and training, resources, and tools.
- Improve access to, and the quality of, data on substance use, pain, and related health and wellbeing factors to maximize their potential for use in local decision-making.
2. What is a T/NASO?
T/NASO means Tribe or Native American Serving Organization. This refers to federally or state recognized Tribes and organizations with a core mission to serve and/or track record in serving Native Americans and that are poised to reach this population. For the purposes of this Program, Native American is inclusive of American Indian, Alaska Native, and Native Hawaiian communities.
3. What is an Ally Organization?
Ally organizations are organizations with the ability to document a track record of partnering successfully with Native American communities. Examples include higher education institutions, local governments, non-profit and for-profit organizations.
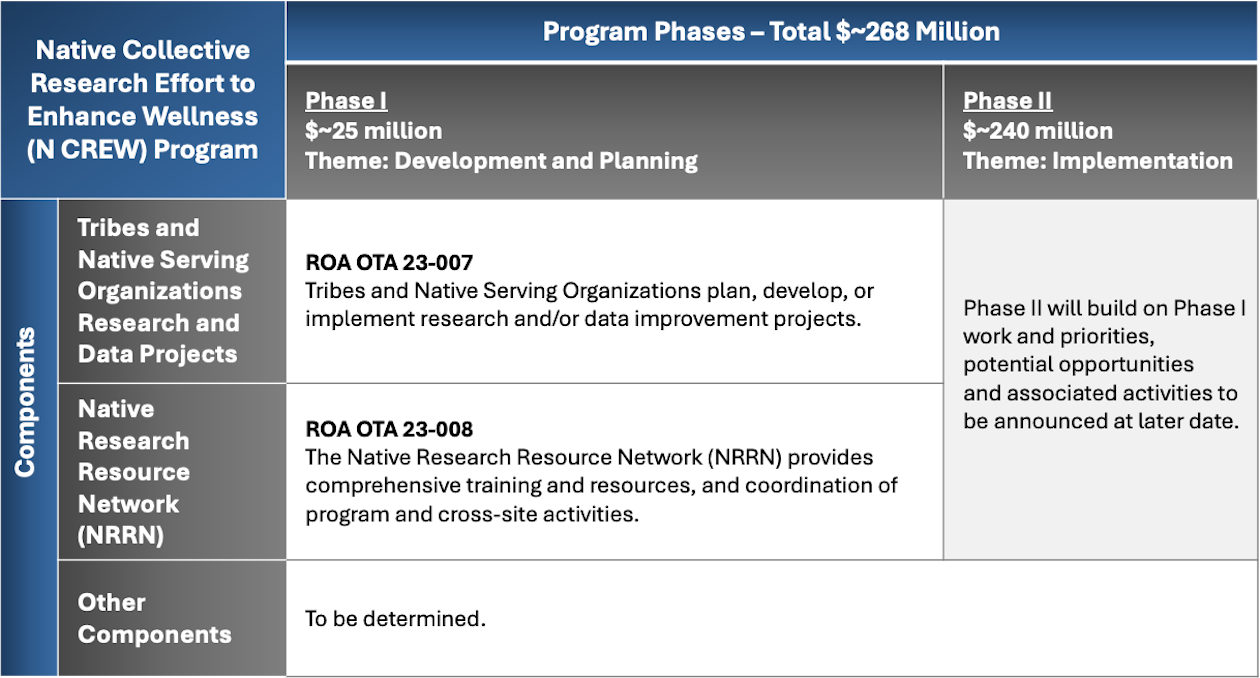
4. N CREW is described on the website as “multi-component” and in the ROAs as a “two-phase” Program. What does that mean?
In short, N CREW is both multi-component in its structure and two-phase in terms of the timing of activities.
Let’s break that down:
N CREW is multi-component in structure.
Throughout the duration of the N CREW Program, NIH will fund projects from two kinds of applications, each engaged in different kinds of activities. Specifically, NIH will fund:
Tribes and Native American Serving Organizations (T/NASO) who want to plan for or conduct research and/or data projects in their community.
And, separately
- T/NASOs and Ally Organizations with demonstrated cultural expertise and experience to provide comprehensive training and resources to support T/NASOs awarded through the application type noted above.
NIH may also introduce other components of the N CREW Program to support achieving the broad N CREW Program goals.
N CREW is two-phased in its timing of activities.
Because the aim of the Program is to build capacity of T/NASOs for engagement in research, NIH has designed the Program in two phases.
Years 1 and 2 constitute Phase I, otherwise known as the planning phase of the Program.
Years 3 – 7 constitute Phase II of the N CREW Program. Phase II will build on what is learned and achieved in Phase I. This Phase will likely support the implementation of research and/or data improvement projects developed in Phase I. Phase II might support additional pilot and development work. The receipt of an award in Phase I does not guarantee recipients’ continuation to Phase II. NIH anticipates Phase II funding will be an open competition, meaning that any organization that meets the eligibility criteria would be able to apply. Phase II funding opportunities are not restricted to Phase I award recipients. NIH will release more information about Phase II at a later date.
The following table summarizes the Program components and timeline:
5. The work we are doing in our community aligns with the goals of the N CREW Program, but we did not apply to the Phase I funding opportunities. How can we get involved?
Please email us at ncrew@nih.gov to let us know about your work! NIH is interested in learning about the different ways communities are addressing overdose, substance use, pain, and related factors. There may be additional opportunities to engage with the N CREW Program.
To receive N CREW resources and information on future funding opportunities, email ncrew@nih.gov to be added to the Listserv.
6. At the outset of N CREW, what were the NIH’s desired outcomes and lessons learned across the Program?
N CREW embodies and recognizes that Tribes and Native American Serving Organizations (T/NASOs) are best positioned to identify and respond to community needs through culturally grounded knowledge and strategies. Therefore, we expect that the Program will lead to more sustainable community solutions stemming directly from the development of culturally-relevant research and data projects and increased capacity for research that is driven by Tribes and Native community priorities. NIH’s desired outcomes for the N CREW Program include the following and understand the outcomes and lessons learned will be community driven:
- Reduce overdose related health inequities through science based, culturally relevant, community embraced, and sustainable strategies
- Discover the best ways(s) to build/enhance HEAL® related research capacity within T/NASOs
- Discover effective/innovative models of Tribal/Native research capacity and infrastructure including opportunities for Native scholars
- Data sharing and stewardship best practices
- Partner to learn under what circumstances (if any) data sharing may be desired and supported
- Potentially build a Data Archive and support cross site collection of outcome data
- Advance application/integration of Indigenous Knowledge in NIH research
Topical Areas of N CREW Research/Data Projects
7. What kinds of research and data projects are funded under the N CREW Program?
Topically the project must include a focus on overdose, substance use (including opioids or stimulants), or pain management. Projects may also include related factors (e.g., well-being, mental health, alcohol use), to support positive health outcomes in Native American communities. Within these topical areas, the specific research and data projects are expected to be diverse since they are applicant-driven and based on community priorities. Learn more about the Phase I awarded projects on NIH RePORTER.
8. Can you provide more information on HEAL related factors?
The N CREW Program is part of the NIH’s Helping to End Addiction Long-term® (HEAL) Initiative to speed scientific solutions to the national opioid public health crisis. The NIH HEAL Initiative® bolsters research across NIH to improve (1) prevention and treatment for opioid misuse and addiction, and (2) pain management. The HEAL Initiative emphasizes a whole-person approach in which individuals and communities are understood as unique, as are risk (e.g., alcohol use) and resilience (e.g., connection to culture) factors related to substance misuse and addiction, mental health, and pain. Further, risk and resilience are understood as intersecting with individual and community-level factors and circumstances. Please keep in mind that funded projects must include a focus on overdose, substance use (including opioids or stimulants), or pain management and may also include related factors (e.g., mental health, wellness, alcohol use). Learn more about the HEAL Initiative.
9. The N CREW Program seeks to promote wellness among Native Americans by advancing research addressing overdose, substance use, pain, and related factors. How is wellness defined?
The N CREW Program does not specify a definition of wellness. Tribes and Native American Serving Organizations (T/NASOs) that apply to conduct research or enhance data as part of the N CREW Program can define wellness based on community priorities.
10. What does it mean that the N CREW Program is a trans-NIH Program?
This means that many Institutes, Offices, and Centers within the National Institutes of Health are in support of the N Crew Program.
Issuing Institutes and Centers include:
- National Institute on Drug Abuse (NIDA)
- National Center for Advancing Translational Sciences (NCATS)
- National Institute of Neurological Disorders and Stroke (NINDS)
The following Institutes, Centers, and Offices are participating:
- Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- National Cancer Institute (NCI)
- National Center for Complementary and Integrative Health (NCCIH)
- National Heart, Lung, and Blood Institute (NHLBI)
- National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)
- National Institute of Dental and Craniofacial Research (NIDCR)
- National Institute of Mental Health (NIMH)
- National Institute of Nursing Research (NINR)
- National Institute on Aging (NIA)
- National Institute on Alcohol Abuse and Alcoholism (NIAAA)
- National Institute on Minority Health and Health Disparities (NIMHD)
- Office of Research on Women’s Health, National Institutes of Health (ORWH)
- Office of The Director, National Institutes of Health (OD)
- Tribal Health Research Office, National Institutes of Health (THRO)
11. Do participating Institutes and Centers have research focus areas for the N CREW Program?
The N CREW Program will support research that is community prioritized to improve outcomes related to the opioid public health emergency, including research and data enhancements related to addiction and/or pain. Within these broad parameters, different NIH institutes each have a different focus. Some examples of what Institutes concentrate on are provided here, but these are examples and are not meant to be limiting.
For example, there are many NIH Institutes, Centers, and Offices focused on pain research and examples of topics of interest include:
- Understanding the biopsychosocial mechanisms of pain with a goal towards therapy development for complex human pain conditions with high unmet needs
- Developing safe, effective, pain therapies and pain management strategies
- Evaluating safety and effectiveness of pharmacological and non-pharmacological approaches, and models of care to improve acute and chronic pain management
- Addressing cross-cutting research areas across multiple ICOs (pain inequities in populations experiencing health disparities; diversity and inclusion in clinical studies; pain comorbidities and under-studied pain conditions)
- Implementing and disseminating evidence-based integrated pain management models of care into clinical practice
As another example, the National Institute on Drug Abuse (NIDA) supports culturally appropriate etiology, prevention, treatment, implementation, and dissemination research focused on opioid or methamphetamine use, alone or in combination with other drugs, among Native adolescents and adults. Specific areas of interest include, but are not limited to:
- Research pertaining to increasing the availability of naloxone
- Intervening to improve the uptake of medication assisted treatment (MAT) to treat opioid use disorder (OUD) or to prevent OUD
- Etiologic or intervention research that addresses substance use (as related to opioid and stimulant overdose) and incorporates comorbid conditions, including alcohol use, suicidal ideation and other mental health conditions
- Research that includes trauma (current and historical), either as an etiological factor or considered in the intervention, including development, implementation, or analysis of trauma-informed interventions to address SUD
While specific areas of focus are not listed for other Institutes, you can find their mission by following the hyperlinks in the previous question.
Funding Information
12. How is the N CREW Program funded?
The N CREW Program is funded through NIH’s Helping to End Addition Long-term® Initiative, or NIH HEAL Initiative®. This Initiative bolsters research across NIH to improve (1) prevention and treatment for opioid misuse and addiction, and (2) pain management. Learn more about the HEAL Initiative. N CREW may use a variety of funding mechanisms, which may include Other Transactions, Cooperative Agreements, Contracts and/or Grants over the course of the Program.
13. What is Other Transactions Authority?
Other Transactions Authority (OTA) is a unique type of authority that allows an agency to enter a legal agreement with a recipient organization that is not a contract, grant, or cooperative agreement. Policies and terms for individual OTs may vary between awards. Each award is therefore issued with a specific governing agreement, which is negotiated with the recipient and may be expanded, modified, partnered, not supported, or later discontinued based on Program needs, changing research landscape, performance and/or availability of funds (Learn more about OTAs on the NIH website).
14. What is different about Other Transactions Authority (OTA) for the N CREW Program?
OTA offers the N CREW Program the unique opportunity to develop a custom Program to support attaining Program goals. This includes supporting the development of individualized solutions in real time. It is a type of funding that allows N CREW to respond to the evolving and unique needs of the T/NASOs that participate in the Program.
More specifically, OTA allows NIH to provide a broad range of research support to award recipients that cannot be fully predicted in advance, meaning that new ideas or needs that arise can be addressed quickly. Because N CREW will connect T/NASOs with partnering organizations to provide the needed support to build T/NASOs research capacity, OTA is ideal for providing flexibility in the partnership selection, development and construct.
Additionally, OTA allows the N CREW Program and participants to quickly pivot. That is, there is agility and flexibility in the ability to modify activities, partners, or formal partnership structures in response to new or evolving needs. This flexibility allows N CREW to support the discovery and creation of the best infrastructure and capacity building strategies possible through, for example, testing novel research infrastructures, bringing in new partners, etc.
Data and Tribal Sovereignty
15. What are the data sharing requirements?
NIH updated its Data Management and Sharing Policy (DMS Policy), effective January 25, 2023. It reinforces NIH's longstanding commitment to making the results and outputs of NIH-funded research available to the public through effective and efficient data management and data sharing practices. Under the DMS Policy, NIH requires researchers to prospectively plan for how scientific data will be preserved and shared. Thus a data sharing and management plan will be required before the time of award. NIH also honors Tribal Sovereignty, and so justified exceptions to the DMS Policy requirements are allowed, as noted in the policy. A data sharing plan should provide a justification for exemptions to the DMS Policy. Please also review supplemental material to the DSM Policy for Tribes (Supplemental Information to the NIH Policy for Data Management and Sharing: Responsible Management and Sharing of American Indian/Alaska Native Participant Data) for additional detail.
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services
