The NIH HEAL Initiative 2025 Annual Report: Research in Action

March 21, 2025
Table of Contents
- Message from HEAL Leadership
- Introduction
- Selected Research Accomplishments
- Advancing therapeutics to address overdose, substance use disorders, and pain
- Optimizing patient care by implementing existing treatments
- Addressing the needs of high-risk populations
- Scientific discovery fueled by shared data
- HEAL Outlook
Message from HEAL Leadership
Now in its eighth year of funding, the Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, is continuing to make progress against the overdose epidemic and the crisis of chronic pain. To date, HEAL has supported research leading to more than 40 investigational new drug and device designations from the U.S. Food and Drug Administration (FDA) to begin human trials for novel treatments for pain, opioid and other substance use disorders, and overdose; driven change in clinical practice for opioid and other substance use disorders within different health care settings and justice systems; and changed the way babies with neonatal opioid withdrawal syndrome (NOWS) are treated. Building on this success, HEAL is extending programs such as the Justice Community Overdose Innovation Network (JCOIN) and launching new ones such as the HEAL PAIN Cohort program, which will advance the field in the long-term by rigorously training early career clinical pain researchers across scientific disciplines.
As leaders of the HEAL Initiative, we have been heartened by the data indicating a reduction in overdose deaths. After years of persistent rises, the United States overdose rate fell 4% between 2022 and 2023, with provisional overdose death numbers continuing to show monthly declines – recent figures show a 24% drop in overdose deaths over the 12 months ending in September 2024. There has also been good news in the fight against pain with the recent approval from the FDA of the first new non-opioid drug for moderate-to-severe acute non-headache pain in 15 years. At the same time, there continue to be many millions of people living with opioid use disorder (OUD), chronic pain, or both – many of whom have other chronic medical and mental health conditions. We are steadfast in our commitment to advancing research that will effectively prevent and treat substance use disorders, overdose, and both acute and chronic pain.
This year, we have seen significant successes supported by HEAL as demonstrated by the start of the second phase of JCOIN, which has already demonstrated that access to medications for opioid use disorder (MOUD) and overdose reversal medications in jails and prisons prior to re-entry reduces mortality, recidivism, emergency service utilization, and increases treatment engagement at re-entry, in a cost-effective way. The HEAL-supported Hemodialysis Opioid Prescription Effort (HOPE) Consortium Trial to Reduce Pain and Opioid Use in Hemodialysis has concluded with good news for people with dialysis-dependent kidney failure who struggle with pain – showing a modest but significant benefit to participants who partook in a pain coping skills program that lessened the interference of pain in their lives. These are just a few of the many HEAL accomplishments described in the accompanying report. In the year ahead, we are looking forward to working with researchers, people with lived and living experience, and community partners to build upon the significant progress already made possible by HEAL-funded investigators to improve the lives of all individuals affected by overdose, substance use, and pain.
Nora Volkow, M.D.
Director, National Institute on Drug Abuse; Scientific Director, NIH HEAL Initiative
Walter Koroshetz, M.D.
Director, National Institute of Neurological Disorders and Stroke; Scientific Director, NIH HEAL Initiative and Co-Chair for HEAL Pain Research
Helene Langevin, M.D.
Director, National Center for Complementary and Integrative Health, Co-Chair for HEAL Pain Research
Lindsey Criswell, M.D., M.P.H., D.Sc.
Director, National Institute of Arthritis and Musculoskeletal and Skin Diseases, Co-Chair for HEAL Pain Research
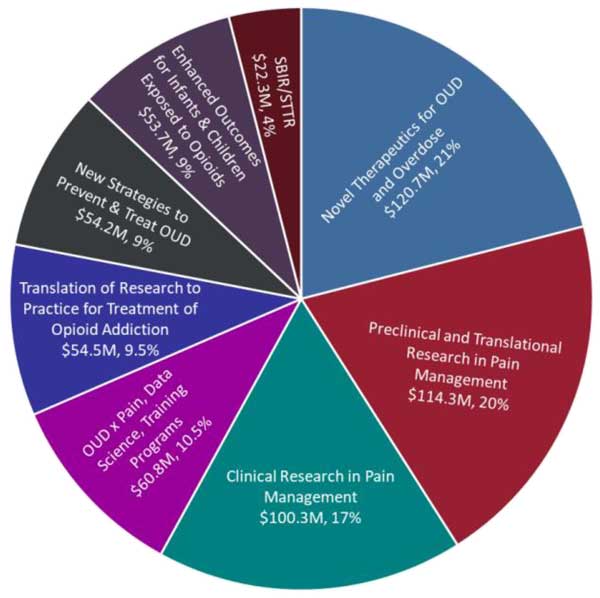
Figure 1. Chart depicting Fiscal Year (FY24) HEAL spending by type of research. Data based on HEAL research projects funded in FY24 as accessed on January 17, 2025. OUD, Opioid Use Disorder; SBIR/STTR, Small Business Innovation Research/Small Business Technology Transfer. (see long description below)
Introduction
The National Institutes of Health (NIH) Helping to End Addiction Long-term® Initiative, or NIH HEAL Initiative®, is an NIH-wide initiative to address the ongoing public health crises of overdose involving opioids, and chronic pain. This initiative, first established in 2018, brings to bear the collaborative research capacity of 19 Institutes, Centers, and Offices (ICOs) at NIH to strategically fund innovative solutions to the rapidly evolving challenges of the overdose crisis while working to better understand, treat, and prevent pain and addiction.
A major aim of HEAL is to address the critical needs of millions of Americans living at risk for addiction and overdose. For several years, the persistently and tragic high number of overdose deaths has been driven largely by the proliferation of cheap, potent synthetic drugs like fentanyl. In 2023, more than 105,000 people in the United States (U.S.) died from a drug overdose, according to the Centers for Disease Control and Prevention (CDC), a 4% decline in overall drug overdose deaths compared to 2022. More recent CDC provisional data show a 24% drop in overdose deaths over the 12 months ending in September 2024. The causes of this decline remain unclear, with possibilities ranging from reduced supply and purity of fentanyl in the illicit drug markets, to increased naloxone availability and expanded use of medications to treat opioid use disorder (MOUD). It is important to fully evaluate and understand the root causes of this decline while continuing to address the radiating impact of the unacceptably high loss of life to drug overdose. As such, HEAL will continue to support research that tackles the many facets of opioid use disorder and overdoses, employing every tool available to design and conduct impactful research that will produce evidence-based scientific solutions for the prevention and reversal of overdose and for treating OUD, which constitutes the main risk for overdoses.
HEAL is also taking on the incredibly complex challenge of chronic pain, a condition that affects over 50 million adults in the U.S. Although pain is a symptom of many conditions, chronic pain is also considered a disease. Nearly 20 million people live with disabling “high-impact chronic pain.” Chronic pain is a biopsychosocial disease that affects every aspect of a person’s life. HEAL studies address all facets of chronic pain, including developing new non-addictive medications and non-pharmacological treatments to safely and effectively manage pain.
Now entering its eighth year of funding, HEAL has driven new and innovative solutions by promoting collaborative science, performing research to improve patient care, integrating shareable data, and pushing the boundaries of rapid therapeutic development. The expansive HEAL research portfolio contains more than 220 funding opportunities, more than 2200 projects conducted by researchers in all 50 states, and a cumulative $3.9 billion investment since its inception. These projects span the gamut of research types as depicted in Figure 1. HEAL endeavors to support research that is truly representative of the U.S. population and that makes a real difference in people’s lives across America. An important feature of the overall HEAL Initiative and many of its individual research programs is the inclusion of the perspectives of community members and people with lived and living experience of addiction and pain in its research studies and programs.
Understanding and treating both pain and addiction are multidisciplinary endeavors that require cooperation and expertise from many different scientific areas. As such, HEAL is closely coordinated and informed by joint leadership from the National Institute on Drug Abuse (NIDA) and the National Institute of Neurological Disorders and Stroke (NINDS) – in collaboration with the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Center for Complementary and Integrative Health (NCCIH) – along with input from many partnering NIH ICOs. In the day-to-day program leadership and management, virtually every NIH Institute and Center is involved in some capacity, with 19 ICOs leading and managing different HEAL programs. In addition, the Multi-Disciplinary Working Group (MDWG) serves as a broad fact-finding body of experts external to NIH who provide input to advise HEAL how to accomplish its ambitious public health mission most effectively. Furthermore, HEAL recently engaged a broad range of constituents—including hundreds of thought leaders and experts, people with lived and living experience, healthcare providers, biomedical researchers, and key partners across the federal and non-federal space—to solicit input on HEAL research priorities. Their input will inform the future of HEAL research aimed at speeding scientific solutions for the addiction and overdose epidemic, including from opioid and stimulant drugs and their combinations, and the significant public health burdens of acute and chronic pain.
Selected Research Accomplishments
Advancing therapeutics to address overdose, substance use disorders, and pain
HEAL funding expands and accelerates the ability of NIH ICOs to support the development of innovative therapeutics for substance use disorders (SUD), pain, and to prevent overdose deaths. Today, the synthetic opioid fentanyl – which is up to 100 times stronger than morphine – dominates the illicit drug supply and has been the primary driver of overdose deaths by itself and in combination with cocaine and methamphetamine, underscoring the critical need for new treatments for SUD and overdose from drug mixtures. Prior to HEAL, NIDA supported a robust portfolio of therapeutics research as exemplified by the development of overdose reversal agents such as naloxone and nalmefene and a pipeline for medication development for SUD that included the development of buprenorphine, the most widely prescribed medication for treating OUD. HEAL has accelerated NIH therapeutic research investments by supporting more than 40 investigational new drug and device designations from the FDA for novel treatments for opioid and substance use disorders, overdose, and pain. In addition, research into the biological underpinnings of pain is paving the way for a new era of non-addictive pain medications – as evidenced by the recent FDA approval of suzetrigine for moderate to severe acute pain.
In 2024, HEAL researchers moved forward with the first clinic-ready, monoclonal antibody against fentanyl, called CSX-1004. This human monoclonal IgG1 antibody treatment binds to fentanyl molecules and prevents them from affecting the brain, preventing and reversing overdose for up to 28 days in animal models. CSX-1004 was also able to reverse overdose of the ultra-potent fentanyl analog, carfentanil, while allowing opioids like oxycodone and methadone to work normally when they are clinically indicated. Based on preclinical and early clinical success, CSX-1004 received FDA fast-track designation – a regulatory designation that expedites the development and review of drugs to treat serious medical conditions and fulfill an unmet medical need.
HEAL researchers are investigating implantable formulations of medications for long-term treatment of OUD and to prevent overdose deaths. Oral formulations that require only weekly administration to treat OUD are already being tested in humans. One formulation, called BIOPIN, slowly releases the opioid-blocking drug naltrexone, providing 6 months of protection against opioid overdose. Furthermore, the Naloxometer is a device that can automatically detect an opioid overdose, deliver life-saving medication, and alert first responders to initiate treatment in a timely manner. This is valuable since many of the overdoses occur when no one is present to administer the naloxone when a person overdoses. Researchers are also testing a non-invasive treatment called low-intensity focused ultrasound to target the reward system in the brains of people with OUD to reduce craving and drug use. In a human pilot study, this treatment rapidly reduced craving with benefits lasting up to 90 days, although larger studies are needed to verify clinical safety and efficacy.
Another promising avenue for treatment of OUD or other SUDs is to identify and repurpose existing therapeutics. Liraglutide, a glucagon-like peptide 1 receptor agonist (GLP-1RA), is an FDA-approved anti-diabetic drug that reduces craving for food similar to other GLP-1RA drugs such as semaglutide (Ozempic). HEAL researchers found liraglutide to also reduce craving in people with OUD in a randomized, double-blinded, placebo-controlled clinical trial study. Such research could lead to GLP-1RAs being a non-opioid based treatment option for OUD. Trials are starting to evaluate second generation GLP-1 drugs to treat OUD.
HEAL research also aims to develop new non-addictive treatments for the millions of Americans who live with chronic pain. Medical providers and the scientific community have long known that taking opioids to treat pain can lead to medication tolerance, requiring more opioid to achieve the same pain relief and even to an increased sensitivity to pain – or opioid-induced hyperalgesia (OIH), sometimes called paradoxical pain. In a study published in 2024, researchers identified a protein called Tiam1 as a key mediator of analgesic tolerance to morphine and of the development of OIH. This promising work could lead to the development of novel therapeutics targeting Tiam1 for achieving pain relief without the side effects of medication tolerance and hyperalgesia.
Treatment options for acute pain also are limited, increasing the risk of developing chronic pain. Recently, the FDA approved a novel non-opioid treatment for an oral, selective sodium channel blocker called suzetrigine to treat moderate-to-severe acute pain. This advance builds on decades of fundamental research on the role of neuronal sodium ion channels in pain signaling; HEAL and several NIH Institutes have funded other foundational work to develop inhibitors of other sodium ion channels (such as NaV1.7) as potential non-opioid analgesics. While this news is heartening, this is just one class of new drugs. Continued investment and support are critical for the development of the many types of non-addictive pain therapeutics that will be required to meet the needs of people in pain. This new approval hopefully will pave the way for a renewed interest from the biopharmaceutical industry in developing non-addictive analgesic therapies.
HEAL also is supporting research into implantable devices as more sustainable treatments for acute and chronic pain. A HEAL-supported group, Allay Therapeutics, received approval from the FDA for their investigational new drug (IND) application to conduct a pivotal Phase II clinical trial for ATX101, an implantable treatment for post-surgical pain following knee replacement surgery. This implant slowly releases a local anesthetic that can provide relief for weeks after surgery and is then broken down naturally by the body.
HEAL-funded research also is delving into the biology behind pain to identify new therapeutics. One molecular target, called MNK, controls a cellular program that makes neurons overactive and produces many of the hallmark symptoms of neuropathic pain following nerve damage. 4E Therapeutics, which is supported by the HEAL Pain Therapeutics Development Program, recently received IND authorization to study an MNK inhibitor (4ET1103) in a Phase I clinical trial for the treatment of neuropathic and rheumatoid arthritis-induced pain.
Support for innovative small businesses is also a critical component of the HEAL investment in developing OUD, overdose, and pain treatments and technologies. This includes the first FDA-approved focused wearable, non-invasive neurostimulation device to assist in the treatment of opioid withdrawal. Based on studies that demonstrated Stochastic Vibrotactile Stimulation (SVS) reduced hyperirritability in opioid-exposed newborns, a small company supported by HEAL licensed the technology developing a bassinet pad that delivers SVS for use in infant incubators and in the hospital setting. Other HEAL supported small businesses have started clinical testing for a new non-opioid drug for neuropathic pain, AFA-281, and wearable pain assessment system, KnowPain. Another company has submitted an IND for a predictive algorithm to assess the personalized risks for maternal relapse and neonatal opioid withdrawal syndrome (NOWS). HEAL funding also supported the successful commercialization of two products: a virtual reality therapy to treat chronic pain and a “Human on a Chip” platform to conduct rapid drug discovery and development.
Optimizing patient care by implementing existing treatments
As HEAL continues the pursuit for additional effective therapies for OUD, overdose reversal, and pain care, there are proven interventions that currently are underutilized in the U.S. HEAL is focused on research that will optimize existing evidence-based treatments and their integration in various community settings – where most Americans seek care – so they can be implemented effectively by health care workers and accessed by patients who need them most.
MOUD, including buprenorphine, methadone, and naltrexone, are the standards of care for OUD, but only about 18% of those who could benefit from MOUD receive treatment. HEAL researchers conducted a comprehensive analysis of MOUD insurance policies across public and private providers and found that coverage for methadone was often excluded by private insurers. In interviews with subject matter experts, researchers identified that rigid requirements for payment from U.S. insurers were an impediment to care. In a separate study, HEAL researchers investigated Medicare and Medicaid eligible peer support services that connect people with SUD to trained peer support specialists. They found these services were often not utilized, particularly for those who were eligible for dual enrollment in Medicare and Medicaid and who could receive the highest benefit from peer support care.
HEAL is uniquely positioned to leverage broad multi-site clinical collaborations to investigate ways to optimize pain and addiction care. In a randomized controlled trial, HEAL researchers found that allowing patients who had a C-section to have a say in how many opioid tablets they were prescribed allowed them to manage their pain while reducing the number of opioids given to each patient. Since unused or unnecessary opioids often are not properly disposed, these data could support a more tailored approach to opioid prescribing that would limit excess opioids from ending up in communities. In another multi-site trial, HEAL researchers demonstrated that telehealth-delivered mindfulness training combined with methadone therapy (MT) versus MT alone significantly reduced drug use, pain, and depression, and improved treatment retention among people with OUD and chronic pain.
Several HEAL-supported clinical trials managed by the NIDA Clinical Trials Network (CTN) evaluated new ways of applying or transitioning between existing MOUD treatments. In the SWIFT trial, investigators found that significantly more patients successfully initiated injectable extended-release (XR) naltrexone using a 5—7-day rapid procedure versus the 12—15-day standard procedure recommended in the medication package insert. The researchers also found that patients required fewer days from admission to first XR-naltrexone injection with the rapid versus the standard procedure, a reduction in hospitalization time with implications for health care cost savings for payors. HEAL researchers have also investigated how to optimize care in emergency department settings, where untreated OUD is often addressed. In a component of the ED-INNOVATION trial, initiating treatment in emergency departments with a 7-day injectable buprenorphine formulation was found to be safe in those with mild opioid withdrawal. This new medication formulation requires fewer visits to care settings and could substantially increase the number of patients with OUD receiving buprenorphine. HEAL researchers have filed an IND application with the FDA to investigate whether a high dose of buprenorphine administered at emergency departments is both safe and more effective than the standard dose. Researchers will investigate if this high dose can better reduce withdrawal symptoms, craving, and illicit drug use for people with moderate to severe OUD.
HEAL also has worked to optimize existing technology used to treat chronic pain with little or no addiction potential. Electrical stimulation of the spinal cord provides relief for a growing number of people with difficult-to-treat severe chronic pain, but its effectiveness has technical limitations. In an initial HEAL-funded clinical study, researchers showed that using an individualized stimulation technology called “high-resolution spinal cord stimulation” has the potential to provide personalized care and more effective treatment of pain. In a separate clinical trial in patients with irritable bowel syndrome, HEAL researchers optimized a non-invasive electrical stimulation technology called transcutaneous electrical acustimulation, which is applied via surface electrodes at specific body locations used in acupuncture to treat pain. Researchers identified optimal stimulation parameters to achieve pain relief and found clues as to how the treatment alleviates pain.
HEAL also has made significant strides in large effectiveness and implementation studies for pain management, reduced opioid prescribing, and overdose reductions. In the largest implementation study launched so far, HEAL researchers assessed the effectiveness of a broad community-based program (among 67 communities across four states). This program tested the impact of an intervention intended to increase access to overdose education and naloxone, MOUD, and safer opioid prescribing practices. Communication campaigns also were developed to mitigate stigma toward use of MOUD, and drive demand for evidence-based care. Though the community-based intervention did not result in a statistically significant reduction in opioid overdose death rates during the evaluation period overall, HEAL researchers reported that the program led to 37% fewer deaths involving a combination of opioids and stimulant drugs other than cocaine. These results underscore the importance of addressing polysubstance use, as fentanyl in combination with psychostimulants continues to be a major driver of the overdose crisis. A major effort within HEAL is to provide evidence for best practices for patient-centered care, including the Pragmatic and Implementation Studies for the Management of Pain to Reduce Opioid Prescribing (PRISM) program. One study assessed the use of electronic health records to collect patient-reported outcomes as a physician learning tool to address patient needs more accurately. Another large pragmatic trial is assessing the effectiveness of acupuncture in older adults over age 65 with chronic lower back pain, which will inform future care coverage by Medicare. And a third study is examining how transcutaneous electric nerve stimulation (TENS) can alleviate the chronic widespread pain of fibromyalgia. Another of the PRISM trials comparing the effectiveness of 2 non-drug strategies for treating back pain at Federally Qualified Health Centers is being expanded via the new CARE for Health™ program in order to reach more individuals where they seek care – in primary care settings.
Resource: Putting Research Into Practice
The HEAL Data 2 Action (HD2A) program employs methods to improve the timeliness and usefulness of data. The research develops innovative, real-world approaches to implement evidence-based interventions to reduce overdoses and improve pain outcomes. HD2A recently released a series of implementation guides and measures to support researchers evaluating strategies in health care and community settings and putting new addiction and pain treatments into practice.
Addressing the needs of high-risk populations
Meaningful engagement of people experiencing overdose, SUD, and pain is essential. Individual HEAL research studies are executed in different settings and contexts, each tailored to enhance engagement of key participants, communities, and other constituents. While the effects of the current overdose crisis are far-reaching across the U.S., certain groups are disproportionately affected and have limited access to needed care and treatment; pain also differentially affects certain populations. HEAL strives to address the needs of all people affected by these conditions by understanding and addressing the unique needs of high-risk populations such as pregnant women and infants, people experiencing co-occurring pain and SUD, and people in recovery. This helps to target resources and interventions for those who need them most.
Babies and mothers
When babies are born with opioid dependence (neonatal opioid withdrawal syndrome or NOWS), they require care to help them through withdrawal. This will typically involve treating newborns with carefully prepared amounts of opioids to treat withdrawal symptoms, but this relies on off-label use of opioids. HEAL researchers recently passed a development benchmark by filing an IND to perform the first safety study of a new formulation of lofexidine (LUCEMYRA®) for newborns. This FDA-approved medication is already used in adults to manage withdrawal symptoms and would provide another option for treating NOWS if proven safe and effective in infants.
HEAL researchers also tested non-opioid strategies to address NOWS. Some of the most common symptoms in babies born with opioid dependence are tremors, excessive crying and irritability, and problems with sleeping and feeding. This new approach called Eat, Sleep, Console (ESC) focuses on these babies’ ability to eat, sleep, and be consoled, and keeps mothers and babies together, enabling families to play a larger role in the care of their infants. While a large multi-site clinical trial last year clearly demonstrated the benefits of this method over standard care, secondary data analysis has revealed new insights this year. When compared with usual care, the ESC approach was associated with less postnatal opioid exposure and shorter hospital stays. ESC care also promoted more breastfeeding in infants with NOWS, which was associated with the reduction in opioid treatment.
The prenatal and postpartum periods are critical development times for both mothers and babies. According to the CDC, roughly 6.6% of pregnant women in the U.S. used prescription opioids during pregnancy with over 20% of those reporting misuse in 2019. In the Outcomes of Babies with Opioid Exposure (OBOE) study, HEAL researchers assessed when pregnant mothers who were taking opioids perceived stigma in their health care settings. Higher perceived stigma was associated with greater depression, anxiety, and anger for mothers in the month following birth. These results show the importance of reducing stigma toward patients and providing trauma-informed care education for health care providers.
People with chronic pain and substance use disorders
Integrative pain management that addresses co-occurring SUD or high risk for opioid misuse requires treatment that addresses whole-patient needs. Through the Integrative Management of Chronic Pain and OUD for Whole Recovery (IMPOWR) Research Network, HEAL provides data harmonization and funding for researchers to conduct 11 unique clinical trials that will investigate integrative care. Protocols for these studies were released last year and describe trials that evaluate new opioid treatment strategies in office settings, MOUD dosage strategies, integrated behavioral treatment, exercise and physical therapy, stress reduction, mindfulness, and telemedicine, among others. By tackling pain care and SUD from many angles and learning which treatment strategies are most effective for this critical group, HEAL is paving the way forward for implementation of better care.
Incarcerated people
Individuals returning to their communities after incarceration face heightened risk of relapse, overdose, and death without timely connection to treatment services. HEAL researchers are actively testing different peer support and patient navigation strategies to facilitate these linkages. In recent publications, HEAL investigators comprehensively evaluated and described these “linkage facilitation” strategies.
While incarcerated people face high rates of OUD, MOUD like buprenorphine is not available in most jails within the U.S. This is partly because of a fear of treatments being diverted in jails for unauthorized use. When interviewing adults who had received MOUD in jail, HEAL investigators found that while MOUD diversion did occur, it occurred less often than assumed, and protocols designed to detect and reduce diversion were recognized as effective. Understanding the issue of diversion, HEAL-funded researchers assessed whether FDA-approved extended-release buprenorphine could be more effectively used in jail-settings and found it viable in a preliminary study. Such studies have helped JCOIN generate real-world data to inform policies and practices to increase access to effective treatments and reduce opioid use and other substance use disorders upon reincarceration. The second phase of JCOIN was launched in fiscal year 2025 with a deliberate focus on the shifting epidemiology of the overdose crisis and a continuing commitment to the prevention of overdose among the high-risk justice-involved population. In the new phase, JCOIN will expand to include research of interventions at earlier intercept points – including crisis response, policing and police deflection programs, and pre-trial services; and research on strategies to systematically scale-up effective treatment practices to a wide range of jurisdictions.
People in recovery
Recovery community centers (RCCs) are a growing resource for people in recovery from SUD. RCCs are designed to foster recovery by providing services directly addressing substance use alongside services that address basic (e.g., access to food) and social (e.g., housing, childcare) needs. Recovery support services can include a variety of non-clinical services to support well-being and clinical continuing care services to address retention on treatment using MOUD. HEAL advances research on the effectiveness of recovery support services by creating multi-constituent networks (researchers, payors, providers, people in recovery) to build infrastructure to set research agendas; build research tools, methods, and relationships; and train researchers needed to generate strong evidence about what works.
In the largest nationwide survey of RCCs to date, HEAL researchers found that by focusing on community-based activities and care, RCCs successfully engage medically underserved populations such as young adults and Black Americans. Researchers also learned that many of the RCCs in the study support MOUD use and engage in supportive conversations with their members about these medications. Additionally, RCCs desire to build collaborative relationships with formal treatment settings and prescribers. Despite the growth of RCCs as part of the recovery support services landscape, they are underutilized.
People with medically complex conditions
People with serious chronic disease often live with pain and struggle to manage these complex medical conditions, including the use of opioid medications, which can have serious negative side effects and carry a risk for addiction. The HEAL-supported Hemodialysis Opioid Prescription Effort (HOPE) Consortium Trial to Reduce Pain and Opioid Use in Hemodialysis completed this year and focused on people with pain also undergoing treatment with hemodialysis for kidney failure. In this large, multi-center, randomized controlled trial, participants underwent a cognitive behavioral pain coping skills intervention delivered by a team of coaches, which had a significant beneficial impact on the extent to which pain interfered with participants’ lives compared with study participants who only received usual care. The study was designed and implemented with extensive input from people who had kidney disease and chronic pain.
Resource: OUD During Pregnancy
The HEAL-funded Prenatal Action for Taking Healthy Steps (PATHS) toolkit offers evidence-based educational resources about the use of medication to treat OUD during and after pregnancy. Resources include videos, discussion guides, fact sheets, flyers, posters, and social media messaging.
Scientific discovery fueled by shared data
Chronic pain and addiction are incredibly complex diseases. A better understanding of their biopsychosocial underpinnings will help speed treatment development and alleviate human burden. One major hurdle to improving treatment is collecting and analyzing high-quality, real-world data to forecast prevention and treatment gaps. As individual research groups generate data from thousands of projects, HEAL has invested in resources that level the playing field so that these data are accessible by all researchers, speeding the path to progress and innovation. Through these efforts, large datasets can be reanalyzed to answer new questions, provide insights for new research directions, and bring researchers together to cooperate on solutions they may not have found otherwise by fostering team science.
To get research results into the hands of investigators and the public, HEAL has invested in an innovative Data Ecosystem. This cloud-based environment supports open science by allowing scientific partners to easily find and analyze HEAL data and research results. This online platform already has over 60 datasets that users can explore and use to answer new questions and glean insights for new research directions. The HEAL Data Ecosystem connects the HEAL community and supports scientific discovery from many researchers, enabling HEAL data to be used to make discoveries and applied to benefit affected communities. As the HEAL Initiative matures and studies conclude, the number of datasets continues to expand rapidly.
As datasets have been getting large, broad, and more complex to analyze quickly, artificial intelligence (AI) data processing through large language models (LLMs) has emerged as a tool for research. In a recent study, HEAL researchers employed LLMs to generate answers to SUD-related questions from social media chatter of people with lived experiences of substance use, which can be a challenging environment for standardizing information. By demonstrating how these LLMs can work, HEAL investigators showed they have the potential to provide researchers with more effective and efficient ways to identify emerging pain and SUD trends using social media and other sources of big data.
Large datasets will help researchers understand the fundamental biology of pain needed to discover new treatments. A major disconnect in pain research lies between the thousands of preclinical studies of pain biology in animals and the dearth of new treatments for people living with pain. To close that gap, HEAL researchers recently published invaluable atlases of sensory neurons from six species. Sensory neurons detect information from the environment and relay it to the central nervous system – the brain and spinal cord. When stimuli are potentially harmful to our tissues, that information is interpreted by the brain as “pain.” These pain-sensing cells, called nociceptors, also are responsible for the aberrant signaling that drives some types of chronic pain which could be targeted for pain relief. But the proteins contained within the nociceptors of humans and mice, for example, may differ. Now, with these atlases, HEAL researchers have generated a powerful resource to help researchers ensure that they prioritize targets that will yield therapeutic benefits in people. Researchers also released four publicly available datasets (on SPARC and Mendeley) with detailed profiles of human pain sensory cell characteristics.
Data from human pain studies also is driving discovery of basic biology. Chronic joint pain is a persistent health issue in the U.S., and pain from osteoarthritis (OA) is among the most common type of chronic pain. Many people with OUD cite joint pain as a contributing factor to their inappropriate use of opioids. Yet researchers’ basic biological understanding of the affected tissues contained within joints (like knee and jaw joints) remains extremely limited. HEAL is supporting researchers in the Restoring Joint Health and Function to Reduce Pain (RE-JOIN) study to map and characterize the sensory neurons and network of nerves that supply the joint and surrounding tissues. New groundbreaking technology also is being developed to provide imaging and other biomarkers for myofascial pain, a common and poorly understood condition that may contribute to a large proportion of back, neck, hip and shoulder pain. As these large research studies are completed, the data will be made publicly available to support a better understanding of musculoskeletal biology and pain and ultimately how to halt or prevent damage to joints and treat musculoskeletal pain.
Large datasets also can help researchers tackle questions that previously have been unanswerable. Healthy brain development is critical to ensuring long-term health in children and infants. However, it has been difficult to understand how exposure to substances and other environmental factors during pregnancy and early childhood affect this development. HEAL is supporting the HEALthy Brain and Child Development (HBCD) consortium of researchers in an ambitious nationwide longitudinal study of infant and childhood development. This year, the consortium published a special issue in Developmental Cognitive Neuroscience describing the study’s goals and objectives, as well as details about its sampling procedures and methods. Importantly, the HBCD consortium anticipates releasing its first datasets to the public in early 2025.
Resource: Supporting the Next Generation of Pain Researchers
In 2024, HEAL made important investments in the future of pain research. For example, efforts to expand and train the pain research workforce have doubled the number of postdoctoral positions at pain research centers. Similarly, HEAL has invested in the National K12 Program which provides support to investigators at institutions without a pain research infrastructure who could benefit from a national mentoring team. A unique feature of both these training programs is that people with lived experience of pain serve as mentors to researchers, grounding the next generation of researchers with a patient-centric perspective from the outset. New training awards are bringing not only early-career researchers but also researchers from other fields and scientific backgrounds into the pain research workforce. Another key component of the pain workforce and training program is the PURPOSE (Positively Uniting Researchers of Pain to Opine, Synthesize, & Engage) network, which aims to connect pain researchers across the continuum of pain research, from all disciplines and at all career stages. The PURPOSE network provides informal training and mentoring opportunities within a specialized social networking platform for pain researchers. Researchers can register and utilize the network by visiting https://painresearchers.com/ .
HEAL Outlook
The NIH HEAL Initiative will continue to drive scientific discovery to address research gaps and barriers to effective prevention and treatment strategies for both overdose and pain – conditions that touch nearly every American. Looking ahead, HEAL will remain focused on identifying the most urgent research priorities to continue to drive the downward trend of overdose mortality and development of new pain management tools and prevention strategies, while providing improved therapeutic interventions and access to care.
Text Description of Figure 1 (above)
Chart depicting Fiscal Year (FY24) HEAL spending by type of research. Data based on HEAL research projects funded in FY24:
- Novel Therapeutics for OUD and Overdose - $120.7M, 21%
- Preclinical and Translational Research in Pain Management - $114.3M, 20%
- Clinical Research in Pain Management - $100.3M, 17%
- OUD x Pain, Data Science, Training Programs - $60.8M, 10.5%
- Translation of Research to Practice for Treatment of Opioid Addiction - $54.5M, 9.5%
- New Strategies to Prevent & Treat OUD - $54.2M, 9%
- Enhanced Outcomes for Infants & Children Exposed to Opioids - $53.7M 9%
- SBIR/STTR - $22.3M, 4%
 U.S. Department of Health & Human Services
U.S. Department of Health & Human Services
